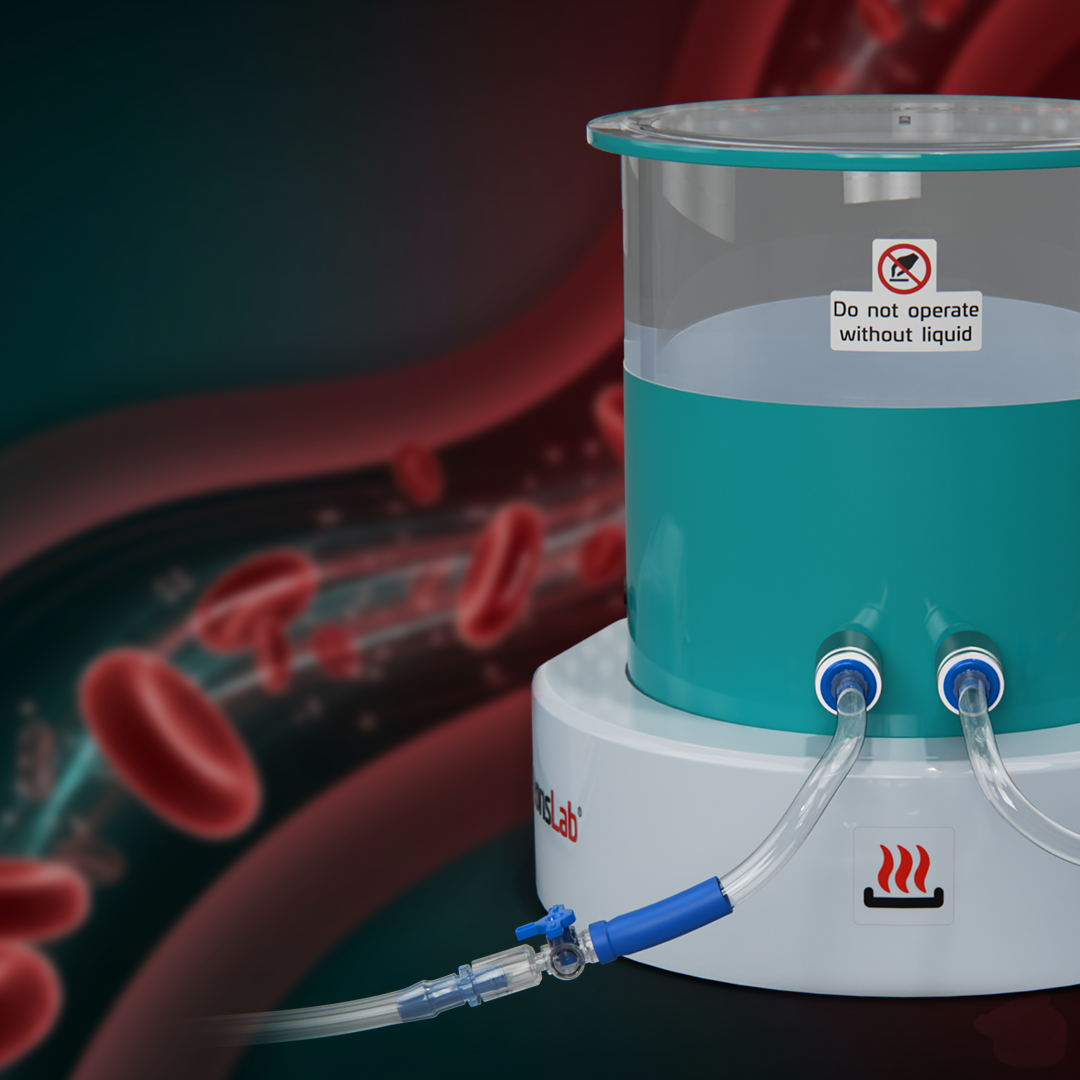
Mini Portable Pulsatile Pump With
Heated Reservoir (37 °C)


At SurgeonsLab, we believe that true-to-life testing and demonstrations are the key to proving the reliability of every endovascular device and implant. That’s why we created the mini portable pulsatile pump with heated reservoir (37 °C) a compact, plug-and-play solution designed to recreate physiologic conditions anytime, anywhere. This portable system is engineered for R & D teams, training specialists, and endovascular companies who need realistic simulation environments for testing, validation, and demonstration.
Setup in minutes and showcase devices like Flow diverters, Coils, Thrombectomy devices, Occluders, IVC filters, and Endografts without heavy infrastructure.
Light weight and travel ready ideal for medical training, R & D labs, conferences, and clinician’s office demos.

Universal Compatibility
Designed to work with embolic devices NBCA, onyx, microspheres, advanced tools like aspiration
catheters and stent retrievers, and covered stents for EVAR and TEVAR.
Confidence in Results
Realistic hemodynamics ensure precise and reliable deployment of closure devices such as PFO
closure, ASD closure, and LAAC—trusted in vascular surgery and interventional radiology.